Page 9 - A HANDBOOK OF ORGANIC ANALYSIS QUALITATIVE AND QUANTITATIVE
P. 9
4 ORGANIC ANALYSIS
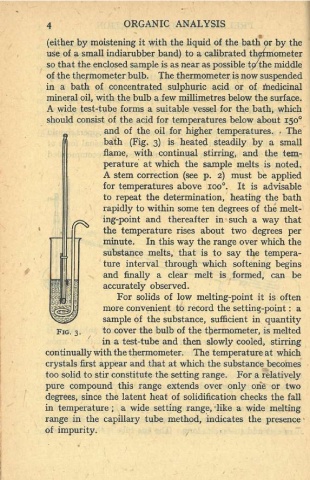
(either by moistening it with the liquid of the bath or by the
use of a small indiarubber band) to a calibrated thermometer
so that the enclosed sample is as near as possible tg'
the middle
of the thermometer bulb. The thermometer is now suspended
in a bath of concentrated sulphuric acid or of medicinal
mineral oil, with the bulb a few millimetres below the surface.
A wide test-tube forms a suitable vessel for the bath, which
should consist of the acid for temperatures below about r50°
and of the oil for higher temperatures. . The
bath (Fig. 3) is heated steadily by a small
flame, with continual stirring, and the tem-
perature at which the sample melts is noted.
A stem correction (see p. 2) must be applied
for temperatures above 1oo. It is advisable
to repeat the determination, heating the bath
rapidly to within some ten degrees of the melt-
ing-point and thereafter in such a way that
the temperature rises about two degrees per
minute. In this way the range over which the
substance melts, that is to say the tempera-
ture interval through which softening begins
d
and finally a clear melt is formed, can be
accurately observed.
For solids of low melting-point it is often
more convenient to record the setting-point: a
sample of the substance, sufficient in quantity
F. 3. to cover the bulb of the thermometer, is melted
in a test-tube and then slowly cooled, stirring
continually with the thermometer. The temperature at which
crystals first appear and that at which the substance becomes
too solid to stir constitute the setting range. For a relatively
pure compound this range extends over only one or two
degrees, since the latent beat of solidification checks the fall
in temperature; a wide setting range, like a wide melting
range in the capillary tube method, indicates the presence
of impurity.