Page 68 - NATIONAL INSTITUTES OF HEALTH (NIH) GUIDELINES FOR CONDUCTING RESEARCH IN MINISTRY OF HEALTH (MOH) INSTITUTIONS & FACILITIES 3RD EDITION 2021
P. 68
APPENDIX 4: EXPLANATORY NOTES ON ADMINISTRATIVE REQUIREMENTS OF
DOCUMENTS SUBMITTED FOR MREC INITIAL ETHICAL REVIEW
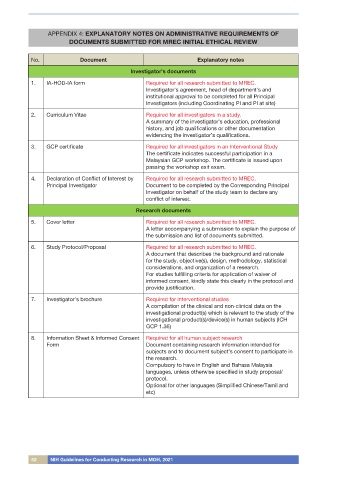
No. Document Explanatory notes
Investigator’s documents
1. IA-HOD-IA form Required for all research submitted to MREC.
Investigator’s agreement, head of department’s and
institutional approval to be completed for all Principal
Investigators (including Coordinating PI and PI at site)
2. Curriculum Vitae Required for all investigators in a study.
A summary of the investigator’s education, professional
history, and job qualifications or other documentation
evidencing the investigator’s qualifications.
3. GCP certificate Required for all investigators in an Interventional Study
The certificate indicates successful participation in a
Malaysian GCP workshop. The certificate is issued upon
passing the workshop exit exam.
4. Declaration of Conflict of Interest by Required for all research submitted to MREC.
Principal Investigator Document to be completed by the Corresponding Principal
Investigator on behalf of the study team to declare any
conflict of interest.
Research documents
5. Cover letter Required for all research submitted to MREC.
A letter accompanying a submission to explain the purpose of
the submission and list of documents submitted.
6. Study Protocol/Proposal Required for all research submitted to MREC.
A document that describes the background and rationale
for the study, objective(s), design, methodology, statistical
considerations, and organization of a research.
For studies fulfilling criteria for application of waiver of
informed consent, kindly state this clearly in the protocol and
provide justification.
7. Investigator’s brochure Required for interventional studies
A compilation of the clinical and non-clinical data on the
investigational product(s) which is relevant to the study of the
investigational product(s)/device(s) in human subjects (ICH
GCP 1.36)
8. Information Sheet & Informed Consent Required for all human subject research
Form Document containing research information intended for
subjects and to document subject’s consent to participate in
the research.
Compulsory to have in English and Bahasa Malaysia
languages, unless otherwise specified in study proposal/
protocol.
Optional for other languages (Simplified Chinese/Tamil and
etc)
52 NIH Guidelines for Conducting Research in MOH, 2021