Page 34 - INSTITUTE FOR MEDICAL RESEARCH ANNUAL REPORT 2020
P. 34
18 IMR Annual Report 2020
Molecular Thalassemia
Molecular tests offered for the diagnosis of thalassaemia are the β amplification refractory
mutation system (ARMS), β GAP, and ARMS variant for haemoglobin E (HbE) thalassaemia. The
laboratory also offers additional panels for thalassaemia detection including:
Cancer Research Centre (CaRC) • α GAP
•
α ARMS
•
α and β Multiplex Ligation-dependent Probe Amplification (MLPA)
α-, β- and δ-sequencing
•
•
A few modifiers i.e., XMN-1 polymorphism and triplication alpha.
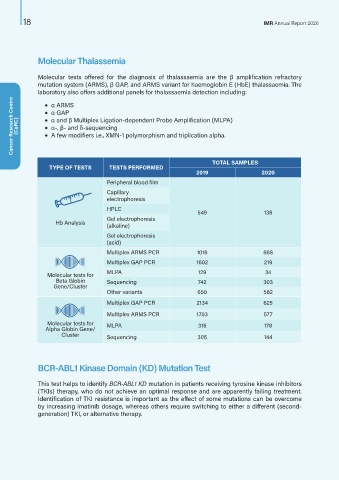
TOTAL SAMPLES
TYPE OF TESTS TESTS PERFORMED
2019 2020
Peripheral blood film
Capillary
electrophoresis
HPLC
549 138
Gel electrophoresis
Hb Analysis (alkaline)
Gel electrophoresis
(acid)
Multiplex ARMS PCR 1016 668
Multiplex GAP PCR 1602 219
Molecular tests for MLPA 129 34
Beta Globin Sequencing 742 303
Gene/Cluster
Other variants 650 582
Multiplex GAP PCR 2134 625
Multiplex ARMS PCR 1703 577
Molecular tests for MLPA 315 178
Alpha Globin Gene/
Cluster Sequencing 305 144
BCR-ABL1 Kinase Domain (KD) Mutation Test
This test helps to identify BCR-ABL1 KD mutation in patients receiving tyrosine kinase inhibitors
(TKIs) therapy, who do not achieve an optimal response and are apparently failing treatment.
Identification of TKI resistance is important as the effect of some mutations can be overcome
by increasing imatinib dosage, whereas others require switching to either a different (second-
generation) TKI, or alternative therapy.